Battery discharged.
The method of discharging the battery has a decisive influence on the durability of the battery, mainly the value of the current drawn and the discharge time.
The discharge of the battery is accompanied by the following (presented in a simplified way) reaction between the plates (active masses) and electrolyte:
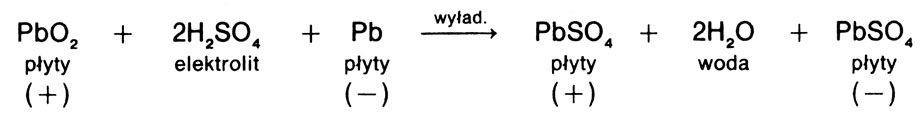
analyzing this reaction, can be explained, why the density of the electrolyte decreases during discharge. Sulphuric acid (dissociated) reacts with the active masses of the plates, resulting in an acidic residue (SO4) is bound to lead dioxide (PbO2) on the positive plate (more precisely, lead), and on the negative plate with lead (Pb) spongy, forming lead sulphate in both cases (PbSO4). At the same time, the amount of water in the electrolyte increases, which reduces its density. The more discharged the cell is, the lower the density of the electrolyte and the more lead sulfate there is on the plates. Continuous undercharging of the cell (battery), e.g. when driving in the city with frequent engine starts and lights on, causes excessive deposition of lead sulphate in the plates. Lead sulfate clumps and clogs the pores of the discs, making it difficult or impossible for the electrolyte to penetrate into them and reducing the capacity of the cell (battery). This leads to sulphation - irreversible changes in the structure of the plates. Normal charging of a sulphated battery does not produce good results, therefore a special type of charging is used - desulphation charging.
The basic condition for the correct operation of the battery is periodic control of the degree of its discharge and appropriate counteraction, to prevent sulphation of the plates, often profound and irreversible.
The battery is most heavily loaded when the engine is started. Under normal conditions, the highest current is drawn from it (so-called. starting current). At this time, chemical reactions in the active masses and the electrolyte take place very rapidly (this is very bad for battery life).
The current drawn by the starter is not always the same value. It depends on the value of mechanical resistance (friction), that affect the starter rotor. The smallest current the starter draws then, when idling (in bulk), i.e. when it rotates without driving any device (a case unheard of in a vehicle). The biggest, when its shaft is fully locked. The starter then flows through the so-called. short-circuit current (in the event of a vehicle breakdown, e.g. engine seizure). Between these current values lies the current of normal operation of the starter. Worth knowing, that the starter reaches its maximum power at exactly half the short-circuit current. Means, that with proper energy use of the starter, the starting current should reach this value.
Remarks. Frequent use of the starter motor when the battery is constantly undercharged leads to rapid sulphation of the battery cell plates.
Starting the car engine with a starter motor should be as short as possible. It should be in the interest of every driver, that all assemblies and systems cooperating with the engine are efficient.
Due to the value of the current charging the battery when starting the engine, it is worth remembering about:
• pressing the clutch pedal (especially in winter), so as not to additionally burden the starter with the frictional resistance of the oil in the gearbox,
• limiting the current drawn from the battery during start-up by receivers other than the starter motor, especially headlights.
Characteristic values of currents drawn by starters of selected domestic cars.
| the car brand | Starter rated power [W] | Characteristic currents [A] | ||
| idling | start-up | short circuit | ||
| Polish FIAT 126P | 500 | 25 | 140 | 235±10 |
| FIAT 127P, 128P | 800 | 30 | 170 | 315 |
| Flag 1100P | 800 | 30-40 | 170 | 290 ±10 |
| FSO 125P 1300/1500 | 1500 | 50 | 300 | 575 |
| Polish | 1500 | 65 | 270 | 540 ±20 |
